Each vaccine needs to be stored in a cool and refrigerated place to ensure its efficiency and effectiveness are maintained during use.
Every year, millions of people are vaccinated to strengthen their immunity and protect themselves from infectious diseases. According to the regulations of the World Health Organization (WHO), vaccination is an approved tool for dealing with deadly contagious diseases that kill 3 million people worldwide.
Vaccines have antigens similar to viruses that cause chickenpox, mumps, and measles. For instance, the influenza vaccine may contain the genetic code of certain strains. The antigens from this vaccine are either weak or dead, so they do not cause diseases, unlike the virus. However, they are effective enough to trigger an immune response that produces antibodies.
The Centers for Disease Control and Prevention or CDC, claims that due to proper storage and handling, the incidence of vaccine-preventable diseases has declined. Proper storage and handling are very important because any errors that occur during this process can cost healthcare providers a lot of time and fortune. Inevitably, these errors result in the loss of patient’s trust, useless doses, and re-application of vaccines. For the healthcare industry, this issue is a challenge because they need to provide vaccine doses on a regular basis.
What Happens If Vaccines Are Not Stored Properly?
All vaccines are biological substances that are sensitive to temperature. If they are not handled or stored properly, they will gradually lose their ability to provide protection against diseases. They are created to affect the immune system, but if they are exposed to temperatures outside the recommended storage range, they will stop working effectively. Once the potency of a vaccine is lost, it will not revert back to its previous state. In addition, there are several vaccines that are extremely sensitive to strong light, hence, they must be kept in a dark place as far as possible. A high level of care must be given to these vaccines as the loss of potency due light to exposure is also permanent and immutable.
Take note that every loss of potency is accumulative. This means that each time the vaccine is exposed to too much heat, cold or light, its efficiency will also decrease. If the vaccine has been exposed to this kind of condition before, then any new exposure, no matter how small it is, will subsequently increase the damage to the vaccine. Eventually, due to cumulative damage, the vaccine is destroyed and becomes useless.
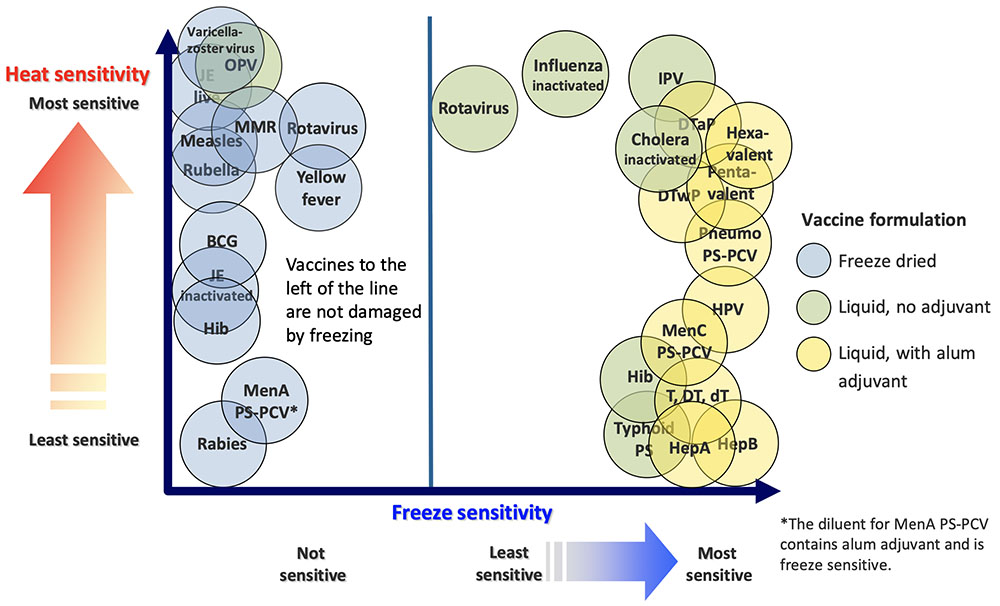
If the vaccine is ineffective, it can pose many threats, including the inability to adequately protect patients from diseases that are expected to be preventable by vaccination.
Which Vaccines Should Be Refrigerated?
The American Storage and Handling have presented a vaccine storage chart:
|
Vaccine |
Where To Store |
Acceptable Temperature Range |
Diluent Storage |
Diluent Temperature Ranges |
|
All DTaP vaccines (DTaP-Hep B-IPV – Pediarix, DTaP-IPV – KINRIX, DTaP-Hib-IPV – Pentacel |
Refrigerator Do not freeze or expose to freezing temperatures |
2°C – 8°C (35.6°F – 46.4°F) |
For those with Diluent, Refrigerator |
2°C – 8°C (35.6°F – 46.4°F) |
|
Hib vaccines (PedvaxHIB and Comvax, ActHIB, Hiberix)
|
Refrigerator Do not freeze or expose to freezing temperatures |
2°C – 8°C (35.6°F – 46.4°F) |
For those with Diluent, Refrigerator |
2°C – 8°C (35.6°F – 46.4°F) |
|
Hep A: Havrix, VAQTA Hep B: Engerix-B, Recombivax HB HepA-Hep B: Twinrix |
Refrigerator Do not freeze or expose to freezing temperatures |
2°C – 8°C (35.6°F – 46.4°F) |
No diluent |
|
|
Gardasil |
Refrigerator Do not freeze or expose to freezing temperatures |
2°C – 8°C (35.6°F – 46.4°F) |
No diluent |
|
|
LAIV: FluMist |
Refrigerator Do not freeze or expose to freezing temperatures |
2°C – 8°C (35.6°F – 46.4°F) |
No diluent |
|
|
IIV
|
Refrigerator Do not freeze or expose to freezing temperatures |
2°C – 8°C (35.6°F – 46.4°F) |
|
|
|
MMR |
Refrigerator or Freezer |
-50°C to +8°C (-58°F to +46.4°F) |
Refrigerator or room temperature Do not freeze or expose to freezing temperatures |
2°C–8°C (35.6°F – 46.4°F) or
20°C–25°C (68°F – 77°F) |
|
Meningococcal Conjugate Vaccines, Menveo and Menactra |
Refrigerator Do not freeze or expose to freezing temperatures |
2°C–8°C (35.6°F – 46.4°F) |
For those with Diluent, refrigerator |
2°C–8°C (35.6°F – 46.4°F) |
|
MPSV4: Menomune |
Refrigerator Do not freeze or expose to freezing temperatures |
2°C–8°C (35.6°F – 46.4°F) |
Refrigerator
|
2°C–8°C (35.6°F – 46.4°F) |
|
PCV13: Prevnar 13 PPSV23: Pneumovax 23 |
Refrigerator Do not freeze or expose to freezing temperatures |
2°C–8°C (35.6°F – 46.4°F) |
|
|
|
IPV: IPOL |
Refrigerator Do not freeze or expose to freezing temperatures |
2°C–8°C (35.6°F – 46.4°F) |
|
|
|
RV1: ROTARIX or RV5: RotaTeq |
Refrigerator Do not freeze or expose to freezing temperatures |
2°C–8°C (35.6°F – 46.4°F) |
For Rotarix, store diluent separately at room temperature
|
20°C–25°C (68°F – 77°F)
|
|
Td: DECAVAC DT: Diphtheria and Tetanus Toxoid and Tdap: Tdap: Adacel, Boostrix |
Refrigerator Do not freeze or expose to freezing temperatures |
2°C–8°C (35.6°F – 46.4°F) |
|
|
|
VAR: Varivax (chickenpox) zoster/shingles) |
Freezer Vaccine should be stored only in freezers or refrigerator/freezer units with separate compartments and exterior doors |
-50°C to -15°C (-58°F to 5°F)
|
Refrigerator or room temperature Store separately from vaccines. |
2°C–8°C (35.6°F – 46.4°F)
or
20°C–25°C (68°F – 77°F)
Do not freeze diluent or expose to freezing temperatures. |
|
MMRV: ProQuad |
Freezer Vaccine should be stored only in freezers or refrigerator/freezer units with separate compartments and exterior doors |
-50°C to -15°C (-58°F to 5°F)
|
Refrigerator or room temperature Store separately from vaccines. |
2°C–8°C (35.6°F – 46.4°F)
or
20°C–25°C (68°F – 77°F)
Do not freeze diluent or expose to freezing temperatures.. |
|
Zostavax (herpes zoster/shingles) |
Freezer Vaccine should be stored only in freezers or refrigerator/freezer units with separate compartments and exterior doors |
-50°C to -15°C (-58°F to 5°F)
|
Refrigerator or room temperature Store separately from vaccines. |
2°C–8°C (35.6°F – 46.4°F)
or
20°C–25°C (68°F – 77°F)
Do not freeze diluent or expose to freezing temperatures. |
How Should Vaccines Be Stored And Handled?
In order to ensure the effectiveness and safety of the vaccine, proper storage and handling are required. It is important to note that vaccines which ought to be refrigerated should never be stored in a domestic refrigerator because it is only designed to keep foods and drinks cold at home, and is not equipped with locks and temperature controls necessary for medical use.
The following are the basic guidelines required for proper storage settings:
- Each vaccine should have its own designated space that is clearly labeled. Storing “look-alike” and “sound-alike” vaccines next to one another should be avoided (For example, Tdap and Dtap, Hep A and Hep B)
- Vaccines should be kept 2 – 3 inches away from the wall of the storage units and other boxes.
- Stickers should be posted on every storage unit and electrical outlet. It is advisable to plug in only one unit per outlet.
- The probe should be placed in the center of the unit.
- The temperature must be checked and recorded twice a day, or at least once a day.
- The door should be filled with water bottles. It is not recommended to store vaccines on the door. Also, make sure that the doors are closed tightly.
- Vaccine storage units should have routine management performed regularly.
- Avoid storing other medications, medical supplies and vaccines in the same unit. If a separate unit is not available, place the medical supplies below vaccines on a different shelf. Placing items on different shelves helps to minimize medical errors.
The efficacy of the vaccines also depends on the way they are handled. When a vaccine is produced, a minimum immunizing dose (MID) is formed. This MID illustrates the amount of antigen that will be included in the vaccine dose at the expiration date. This helps to convince producers that, if handled correctly, the vaccine will provide protection during its validity period.
It is also imperative for all vaccines to be used immediately once opened. This is the best way to ensure that the product remains effective. If the vaccine is not used immediately, it should be disposed of properly. Also, moving the vaccines that have the soonest expiration date to the front of the unit with a mark, using the vaccines with the soonest expiration date first helps in making sure that they will be picked up first by someone who is selecting the vaccine from the unit.
Inappropriate Temperatures Can Undermine Vaccines
As mentioned above, vaccines must be correctly stored upon production. In addition, health care professionals must keep them within the recommended temperature range or they will lose their effectiveness. The ideal temperature range for vaccines is 2°C to 8°C (35.6°F to 46.4°F).
Medical refrigerators should maintain an average of 5°C (40°F). If the temperature exceeds its range, the problem should be reported to the supervisor immediately so that the dose is kept from being invalid. On the other hand, freezers must be kept at a temperature between -50°C and -15°C (-58°F to +5°F).
Choose Medical Refrigerators As Storage For Vaccines
When storing vaccines, it is best to select the equipment recommended by the CDC to prevent any loss of expensive vaccines or use of damaged vaccines. The CDC suggests stand-alone and self-contained medical refrigerators because these units are designed specifically to store vaccines.
There are various medical refrigeration units available in the market including compact, counter-top, under-the-country, and pharmaceutical grade units. However, studies have found that stand-alone devices can maintain the required temperature better than a combination of domestic freezing/refrigeration devices.
Conclusion
In order to maintain an effective vaccination program, all vaccines must be correctly stored in appropriate cold storage units within a very specific temperature range from the start of production until they are used. Standard refrigerators cannot provide this level of accuracy, which risks the effectiveness of the vaccine and the well-being of the vaccinated. A slight change in the temperature is enough to reduce their effectiveness against vaccine-preventable diseases.
Furthermore, the correct storage and handling of vaccines is a key factor in protecting individuals and communities from certain diseases. In fact, the responsibility of producing high-quality vaccines is shared by everyone, from the time they are manufactured to the moment they are administered. It may sound unfortunate, but the cost of spoiled vaccines and the replacement of vaccinations are very high, especially for the third world and developing countries where vaccines are mostly needed. Hence, proper storage and handling procedures must be maintained to prevent such incidents from happening.
Temperature Monitoring – Pharma-Mon by AKCP
For more information about AKCP’s dedicated pharmaceutical monitoring solution visit www.pharma-mon.com






