All vaccines are sensitive to extreme heat and extreme cold and require both proper vaccine storage and management to avoid any loss of potency. Temperature sensitivity of vaccines is vital to understand.
Immunization is the most effective and economical public health measure against infectious diseases. Immunization prevents two to three million deaths every year from diseases such as hepatitis, pertussis (whooping cough), influenza, and measles. In spite of this, more millions of deaths could be avoided if temperature-sensitive vaccines were transported and stored correctly.
Increasing attention is being paid to vaccine management to prevent vaccines from being exposed to excessive heat and cold. With effective management as the goal, new equipment and training programs are being developed. Immunization managers implement these programs using knowledge of a vaccine’s durability. Particularly the rate of loss of characteristics, and the time of exposure to temperatures ranging outside 2°C to 8°C (35°F to 46°F). This protects the vaccines.
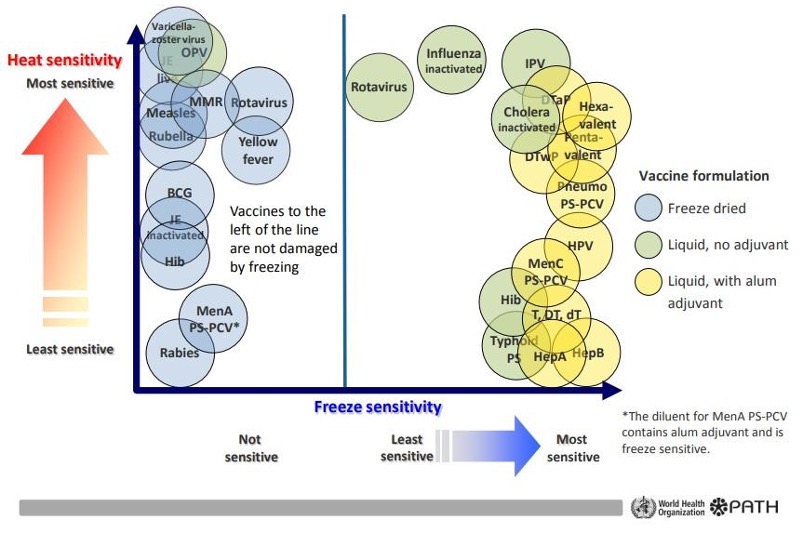
Vaccine Stability: Issues And Implemented Actions
It is inarguable that vaccines protect millions of human lives every year, but because of the temperature sensitivity of vaccines, they can become less effective. Hundreds of thousands still die – especially in developing countries, which often do not have enough effective storage facilities. Various types of instability occur during the manufacture, storage, and distribution of vaccines. Most vaccines have poor thermal resistance and need to be stored at a temperature of 2°C to 8°C (35°F to 46°F) from the start of production to the time of administration to the patients. The main issues along with the corresponding actions related to vaccine stabilization are listed below:
Ensuring the maximum effectivity of the vaccine requires careful attention in handling practices at the country level
Primary vaccine producers and shippers are required to safely carry out the vaccine storage and transportation process. They deliver vaccines from the producers to the end-users at local health facilities. Vaccine management consists of all of the activities that involve the handling of vaccines at the country level. From the moment they arrive, until the moment they will be put to use. These activities include implementing arrival and acceptance procedures, correct monitoring of temperature, checking sufficient storage volume, observing the required standards of buildings, equipment, and vehicles, maintaining efficient inventory management and vaccine delivery systems, using effective policies such as multi-dose delivery systems (MDVP), and utilizing vaccine vial monitors (VVM).
There are standard tools available today from the World Health Organization (WHO) and the United Nations Children’s Fund (UNICEF) to successfully monitor the performance management of vaccine stores. The analysis performed by those tools shows that there are still delicate issues in vaccine management performance which require more attention. The findings from assessments conducted by WHO in the early 2000s pointed to the following areas for improvement:
- Medical or healthcare staff use inadequate vaccine arrival procedures to record the quality of arriving vaccines.
- The staff does not observe correct maintenance of the equipment within the temperature range recommended by WHO and does not take the appropriate follow-up actions when such violations occur.
- Many countries still do not have temperature monitoring devices (TMD) suitable for primary and intermediate stores and lack sufficient cold storage capacity. Some regions have TMDs that are very old and need replacing.
- Inventory management needs improvement. Counterproductive systems cause vaccine expiration during storage, and the facility staff does not constantly practice the “Earliest Expiry First Out” (EFFO) principle.
- Vaccine distribution still puts vaccine quality at great risk. Delivery personnel, especially in developing countries, still carry freeze-sensitive vaccines with frozen ice packs and/or poorly conditioned ice packs, which may expose the vaccines to excessively cold or freezing temperatures.
- The vaccine management culture does not set a standard operating procedure for the handling of vaccines at the country level.
- Financial and human resources are insufficient to offer work support.
The facilities don’t use multi-dose vial policy (MDVP) or vaccine vial monitors
(VVMs) to their maximum potential.
The impact of heat and cold temperature on vaccines is progressive, which is why cold chain manufacturers attach VVMs to their products
Vaccine vial monitors indicate whether a vaccine has been exposed both to excessive heat or cold temperatures over time, and whether they may have been damaged in any way. It clearly shows health workers and staff if the vaccine is fit for usage. Furthermore, VVMs track the vaccine’s heat stability curve, which allows for a margin of safety. They are ideally suited for monitoring temperature sensitivity of vaccines.
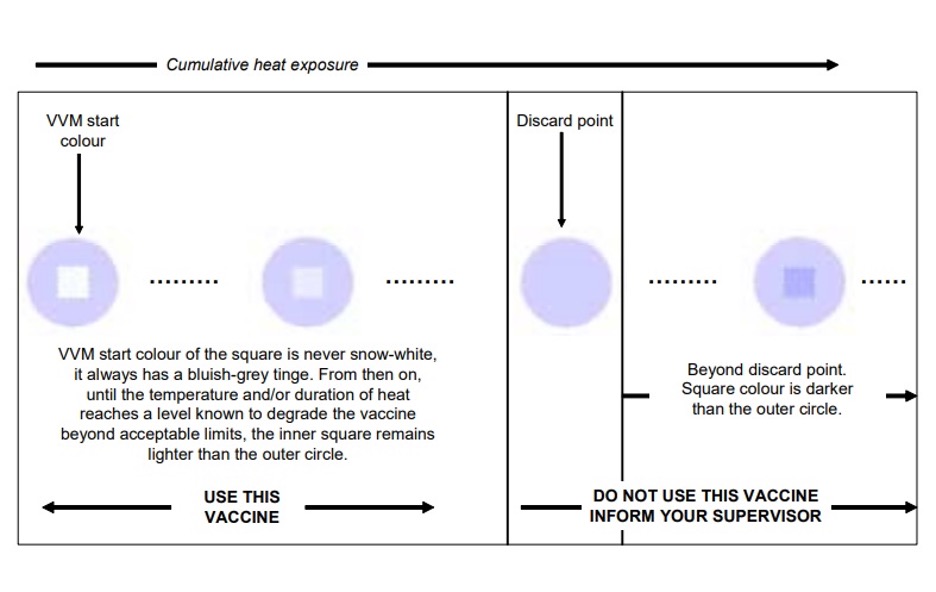
Despite having such strict requirements, failures may still occur. Even vaccines equipped with VVMs are subject to damage if they are removed from the cold chain, and if health workers and staff who manage the vaccines are not skilled enough to interpret VVM readings accurately. If workers disregard the significance of the readings, and a vaccine has experienced excursions, problems will result.
WHO suggests that the use of vaccines outside the cold chain is possible only if necessary guidelines will be implemented for all routine vaccination activities, or on a restricted condition in certain areas or circumstances, such as the following:
- national vaccination days;
- remote areas;
- immunizations administered at home;
- cool seasons;
- storage and transportation of freeze-sensitive vaccines (such as DTP, TT, Hepatitis B, and Hib vaccines) where the risk of reaching a freezing temperature is higher than the risk of getting exposure from excessive heat.
Prevention of freezing becomes a key factor in ensuring the quality of vaccines
There are widespread procedures and practices that expose vaccines to freezing temperatures, at all levels of health systems, both in developed and developing countries. If the vaccines are damaged by being exposed to sub-zero temperatures, loss of potency is considered irreversible.
Freezing affects vaccines by changing the vaccines’ physical form, along with its immunogenicity and texture features. It also affects their structure, whether they are monovalent or combined. The only elements that freezing does not alter are the non-potency parameters, such as acid substance, pH levels, the ability to flocculate, the ratio of free aluminum to aluminum phosphate, free formaldehyde, and thimerosal content.
In 2005, a working group established by WHO introduced a new policy to prevent vaccines from freezing during transportation, that is, using cool water packs. This method replaced the inefficient use of deep-frozen ice packs, which inadvertently expose freeze-sensitive vaccines to freezing temperatures during their transport from one facility to another. According to studies conducted by WHO, cool water packs which are cooled at +2°C to +8°C (+35.6°F to +46.4°F) in the main section of the refrigerator provide a safe temperature margin for all vaccines, except oral polio vaccine (OPV). These packs allow the vaccines to be transported even in hot climates and can be reused up to four times.
As more countries and regions introduce expensive vaccines that can be easily destroyed by freezing, such as combination vaccines, it becomes more important for health and medical managers to identify and implement solutions to such problems. Even though VVMs are designed as time-temperature indicators, they can offer a great deal of help to reduce vaccine freezing. When implementing practices to store and transport vaccines, VVMs allow managers to detect and avoid exposure to excessive heat without using ice or equipment considered to be sources of freezing damage. With the help of these smart devices, health workers will be able to understand that a significant amount of vaccines do not inherently go bad if the power is cut off overnight. Instead, workers will be able to see that freezing poses a greater danger to the thermal stability of vaccines than mild heat exposure.
As technology has advanced, electronic data loggers and realtime monitoring systems have been developed, such as those supplied by AKCP.
Vaccine cold chain systems have been established using a single set of rules
For the past 20 years, the global vaccine supply chain has been maintained by a set of standard vaccine handling guidelines, without considering the needs of the local environment and the types of vaccines required by each country. This approach made the cold chain easy to comprehend, implement, manage, and achieve, but it led to the progressive onset of complacency, that prevented health workers from taking full advantage of the thermal stabilities of various vaccine types. To solve this problem, immunization programs worldwide evolved and diversified. Operational strategies began to expand to remote areas, special campaigns covered a larger target population, and more efforts were made to reach unprotected children.
Vaccines have become more stable, and there is clearly the prospect of improving thermal stability. In these situations, an arbitrary approach to the cold chain causes resources to be wasted and places unnecessary restrictions on the field of operations.
VVMs can be considered as a stimulus for the required changes in vaccine distribution strategies in the cold chain, as they allow immunization programs to monitor the stability of each vaccine to the greatest possible extent, minimize distribution expenses, and increase flexibility in the vaccine handling and management process, hence making operations more efficient.
Inevitable Demands On The Cold Chain
Evolution of the cold chain in the 21st century looks promising, as stated in a document published by WHO in 2000. It states that the factors that will determine the success of vaccine management depend on new and upgraded strategies, vaccines, and tools.
As new vaccines are being developed, this is the best time for immunization managers to communicate with vaccine manufacturers to explore the most thermally stable options available. The Program for Appropriate Technology in Health (PATH), an international nonprofit health organization, is proactively conducting research on vaccine stabilization technologies. They are collaborating with business partners to facilitate studies on the technical
and commercial feasibility of heat-stable vaccines. Their technical research puts more emphasis on sugar-classification and spray-drying based technology applied to the Expanded Program on Immunization (EPI) vaccines. Feasibility studies for measles and hepatitis B vaccines are ongoing, and will affect the technologies together with the antigens that are to be studied in the next round.
Generally, new heat-stable vaccines are monitored for shelf-life storage at moderate room temperatures. The availability of heat-resistant vaccines can improve their immunization effectiveness by reducing the loss of potency due to heat or freezing damage, while minimizing cold chain costs and logistics requirements. Finally, new tools are being utilized which will allow flexibility in vaccine management. These tools include the VVMs mentioned above, as well as other devices that monitor vaccine temperatures in the field.
Conclusion
The stability of vaccines varies widely and can be ranked according to the temperature sensitivity of vaccines. Although some vaccines have heat stability features, other vaccines are more sensitive to changes in temperature. Every time these vaccines are exposed to high temperatures, even if the remaining efficacy of the vaccine is higher than the level considered to be the lowest immune potency, the characteristics of the vaccine still undergo a certain degree of degradation. Moreover, each exposure to ambient temperature has an increasing impact on vaccine efficacy.
All vaccines should be stored routinely at the temperature recommended by the manufacturer and the national immunization programs. The cold chain remains a highly delicate point for all immunization programs. In all countries, refrigeration systems, temperature-monitoring, and record-keeping are required to ensure that each vaccine vial is kept within its appropriate conditions and that the vaccines are utilized before the expiration date shown on the label. These changes demand behavioral adjustments on the part of immunization managers, including better knowledge of the characteristics of the vaccine products they are using. Improved vaccine management policies and procedures, a well-regulated vaccine supply chain, and more attention to the problems related to freezing of vaccines will improve vaccine safety. More focus on the effects of loss of products due to excessive heat or cold exposure will help control monetary losses as the price of vaccines rises.
References
https://apps.who.int/iris/bitstream/handle/10665/69387/WHO_IVB_06.10_eng.pdf?sequence=1
https://www.who.int/immunization/programmes_systems/supply_chain/resources/VaccineStability_EN.pdf
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4599698/
http://vaccineresources.org/files/Vaccine-tech-21st-century.pdf