Temperature Monitoring for Vaccine Storage and Transport
Regulatory requirements and good practice determine that monitoring of your drug storage environment is critical. Many vaccines and drugs require storage between 2°C and 8° (35.6°F to 46.4°F). Some mRNA based vaccines require ultra low temperatures of down to -90°C.
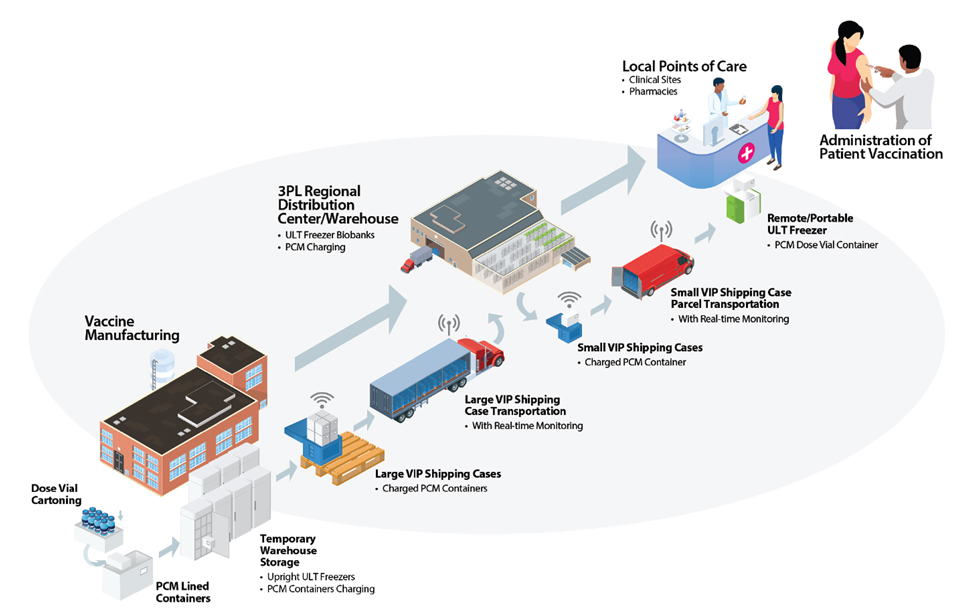
The AKCP pharmaceutical and vaccine delivery monitoring solution can track shipments from factory to delivery, and the end user. Granular visibility of your pharmaceutical cold chain down to individual vial.
Wireless temperature sensors with calibration check and failover act as dataloggers and synch data to AKCPro Pharma-Mon Server.
Vaccine Transport and Storage System
NIST Wireless Temperature Sensor and Data Logger

SP-WT with NIST Temperature Sensor connected
Place the NIST calibrated, certified temperature sensor with internal calibration check and failover on your shipment, inside your warehouse or medical refrigerator. Monitor temperature and log data internally. Anytime the sensor is within range of an AKCP Wireless Tunnel™ Gateway (WTG) data is sychronized and uploaded to AKCPro Pharma-Mon Server.
Install WTG’s at strategic points through your vaccine cold chain. Connect via WiFi, Ethernet or cellular data. For live monitoring during transport, such as inside refrigerated trucks, GPS option is available.
Through this visibility of the whole supply chain you can minimize temperrature excursions and ineffective vaccines.
AKCPro Pharma-Mon Server
Pharma-Mon Server is our IoT cloud based platform for monitoring all deployed sensors in your network. Track vaccines by shipment, carton, or individual vials from factory to warehouse to medical facilities. Monitor temperature, view complete graphs of vaccine history, total accumulated time outside of cold storage.
– Graphs and reports are digitaly signed and in compliance with FDA 21 CFR Part 11.
– Simple drag and drop user interface to transfer shipments through the supply chain
– Granular history of vaccine vials, inventory and alerts.