Importance of Monitoring Pharmaceutical Storage Temperature
Ensuring proper environmental monitoring of pharmaceutical storage temperature conditions is crucial for the stability of the products.
The conditions should be consistent throughout the supply chain logistics. Poor temperature control can be detrimental to the effectiveness of the drugs. Improper storage conditions at the warehouse or in transit can cost thousands in wasted products.
The pharmaceutical and healthcare industries are expected to deliver high-quality products to their patients. Monitoring of the climate in the storage of pharma products is a priority. This ensures the safety and consumer well-being. Controlled storage temperature plays an important role in this.
Monitoring Pharmaceutical Storage Temperature Ranges

Each medication type has recommended storage conditions. Specific storage temperature conditions and ranges are recommended. Adherence to this guarantee their quality and provide the intended efficacy.
There are several categories for the temperature ranges of medicines:
- Non-Refrigerated medicines. Stored ambient room temperature between 10°- 25°C. Usually labeled keep under 25°C, though some are stable up to 30°C
- Refrigerated medicines, including vaccines. Stored between 2°-8°C to maintain efficacy. Strict temperature control is required. Any breach of the cold chain could lead to discarding the pharmaceutical product.
- Freezing temperatures between -10°C to -25°C. Necessary for specific drugs and vaccines such as Zoster, Varicella, or MMR.
Pharmaceutical Products Safety
Improper temperature storage may have different effects on the medications and drugs stored. If temperatures are out of range by a few degrees Celcius their chemical stability can be affected. Worst case it can alter a drug’s physical properties. This can have dangerous consequences when administered to patients.
Live virus vaccines tolerate freezing temperatures but rapidly deteriorate after removal from storage. Damage to inactivated vaccines occurs when exposed to temperature fluctuations. It is crucial that the proper storage and handling are adhered to.
There are specific procedures that should be followed when a violation of storage temperature for vaccines occurs.
- Immediately isolate the vaccines and prepare them for storage between +2°C and +8C.
- During storage label them “Do Not Use”
- Contact the manufacturer for advice
- Determine if anyone has received a compromised vaccine
Monitoring Pharmaceutical Storage Temperature – Best Practice
The W.H.O provides guidelines for good storage practices of pharmaceuticals. It recommends recording the storage temperature data for quality assurance review. It should be kept for at least the shelf-life of the stored product plus 1 year. Some national legislation may require longer.
The below chart shows the storage requirements at different levels of cold chain distribution for Polio Vaccines. Monitoring at each stage, but particularly at stages 1-3 is vital.
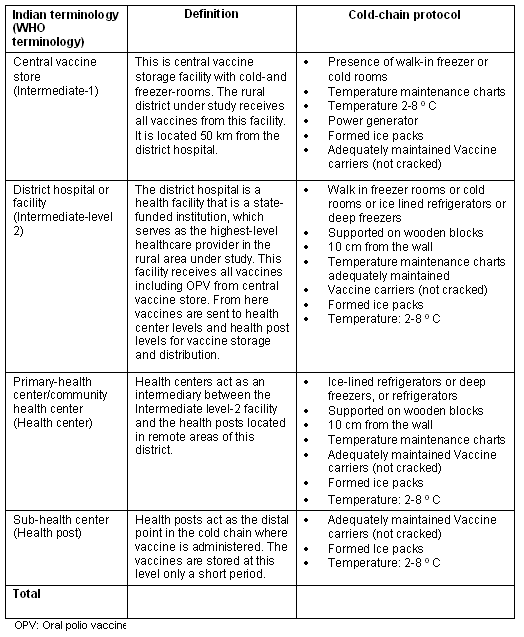
The protocol published by W.H.O recommends a generator backup power for the storage facilities. Monitoring of these backup power systems can also be implemented to ensure readiness when required.
The Australian Government department of health published a chart that should be filled in twice a day. Manual temperature checks and plotting on the chart is a time-consuming and error-prone task. When the temperature is out of range, it is unknown for how long the condition has existed since the last check.
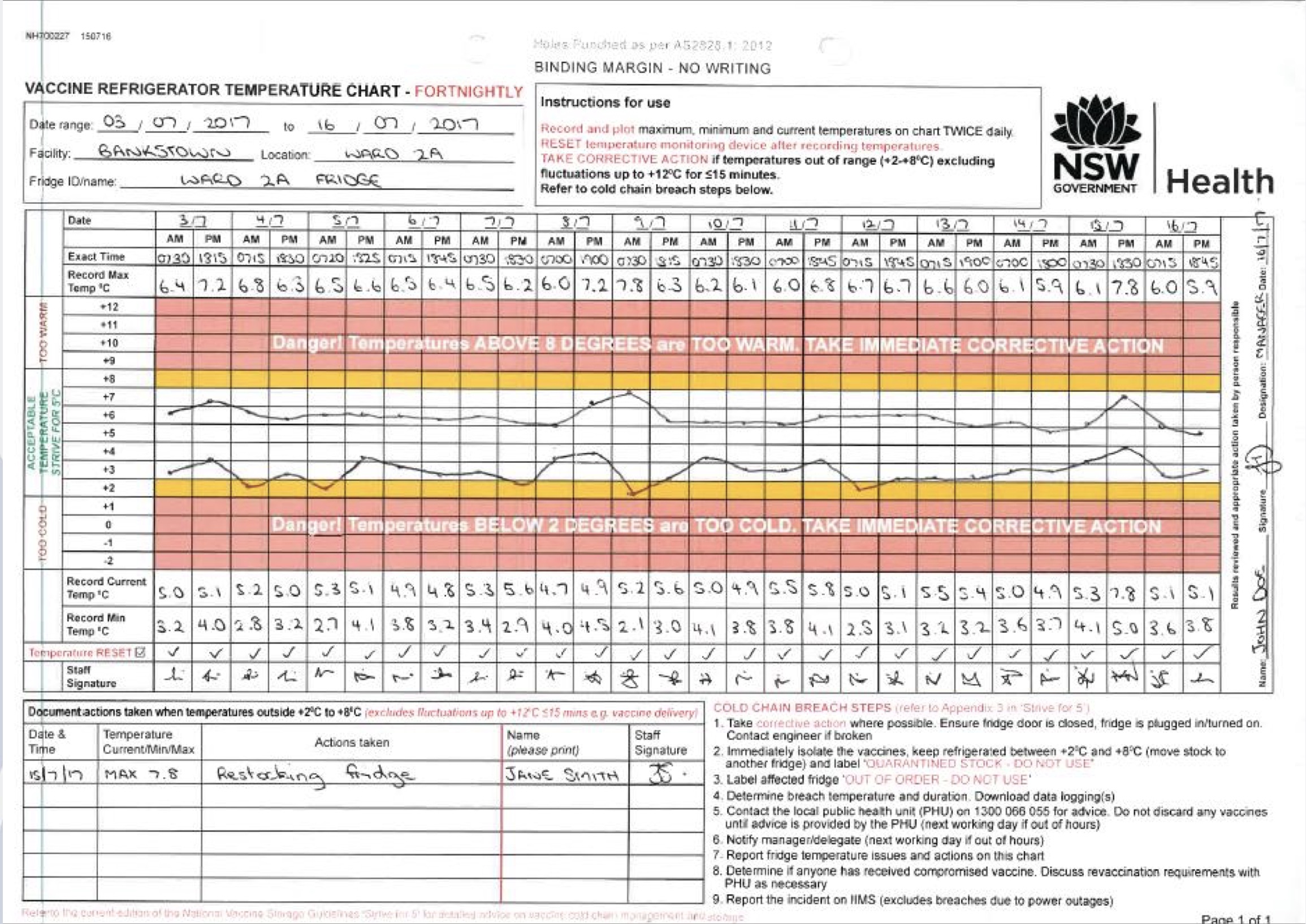
Image courtesy of NSW government
Temperature monitoring systems should be installed to monitor the cold storage environment. The system should also be capable of logging data and producing reports and graphs. Monitoring and alerts when abnormal conditions occur can save thousands in lost products.
Warehouse temperature mapping should show the uniformity of the temperature across the storage facility. Temperature sensors should be located in areas that are most likely to have fluctuations.
The monitoring system can be wired sensors or wireless sensors. When dealing with cold storage, often the wireless option is preferred. Wireless sensors are easy to install and can operate on batteries.
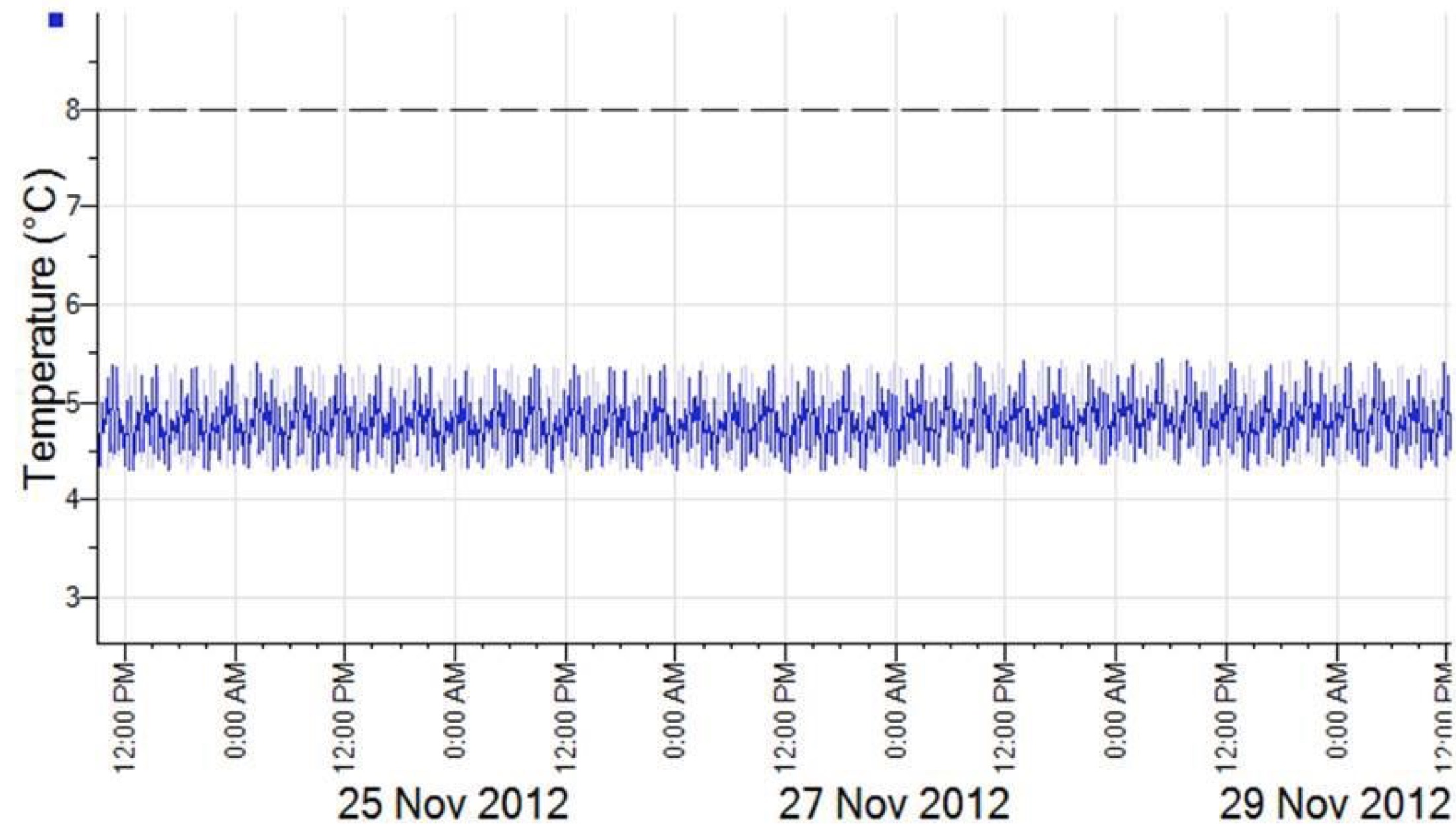
The below charts are an example of data collected from a monitoring system.
The first chart is from a stable refrigerated storage environment. The temperature fluctuates with the compressor cycles but is maintained within the defined limits.
The second chart illustrates a high-temperature breach. This could be caused by a power failure or malfunction of the refrigeration system. A temperature monitoring system would alert once the threshold has been exceeded allowing for immediate response. A power monitoring system on the refrigerator would give an earlier warning of the problem.
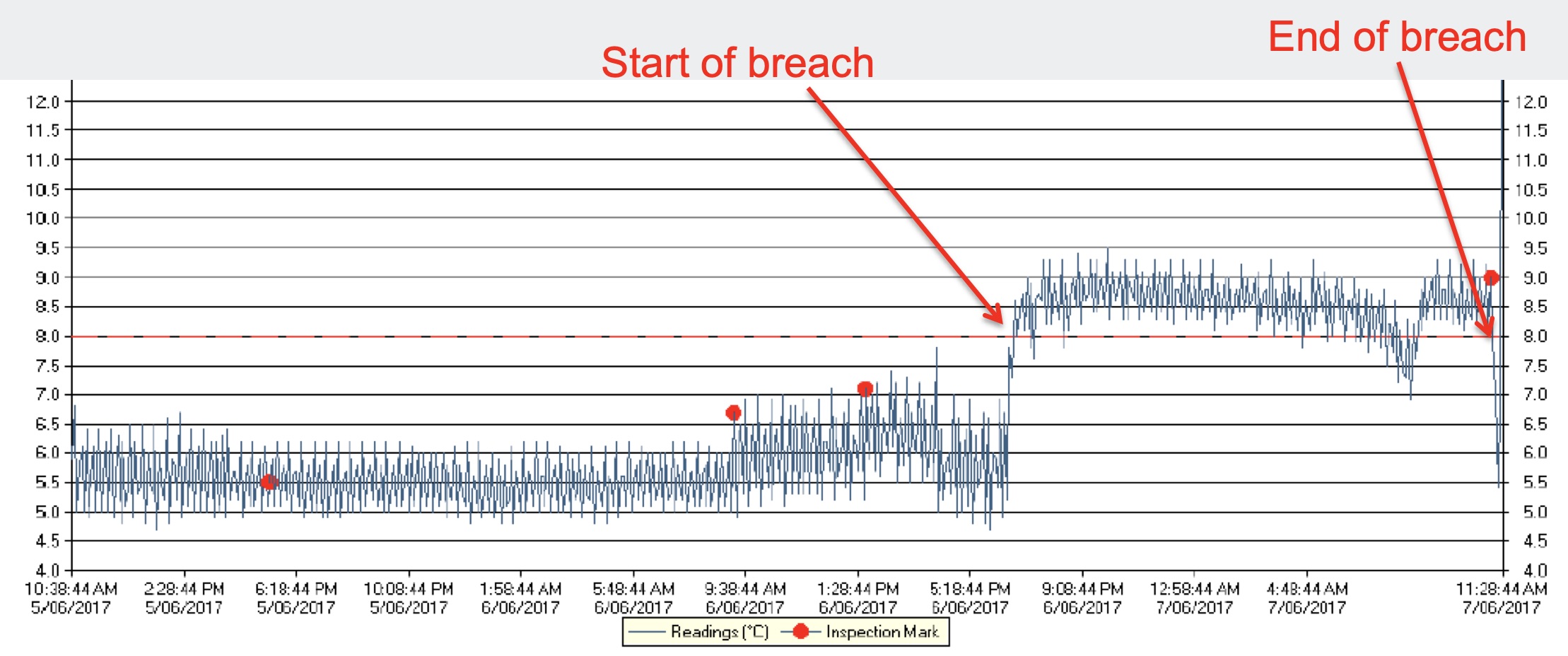
Charts courtesy of NSW government
Monitoring Pharmaceutical Storage Temperature Cold Chain Distribution
A critical time for pharma products is when in transit to their destination. During transport, they could be in an unpredictable and uncontrolled environment. Monitoring during cold chain transport and delivery is also crucial. In some countries, it is mandatory for data logging to take place during transport.

Temperature-controlled logistics are crucial for product quality and minimizing spoilage.
Logging the temperature of refrigerated and frozen medicines during their transit gives traceability. Analyze downloaded data to verify the maintenance of temperature compliance. Temperature data logging gives early detection of degraded lots before reaching patients.
Humidity Control Matters Too
Additional to temperature monitoring, humidity monitoring has a role in keeping pharmaceuticals safe. High humidity can cause pharmaceutical drugs to absorb moisture. This can occur during final packaging, shipment, or storage. causing certain medications to degrade and lose efficacy. Monitoring humidity levels keep drugs stable through to their expiration dates. Mold growth on packaging can also occur n high humidity environments. Medicines should be in a well-ventilated warehouse. Monitoring aids in maintaining humidity levels of 60% or lower.
Pharmaceutical Cold Storage Monitoring Solution
With AKCP’s wireless monitoring solution, drug storage monitoring is rapidly deployed. Realtime alerts, data logging, and reporting comply with regulatory requirements. Wireless Tunnel™ radio technology penetrates even thick secure storage and refrigerated cabinets. Battery-powered sensors with a 10-year battery life guarantee for easy installation.
A monitoring system gives precise knowledge of the temperature and humidity. Real-time monitoring allows you to be confident in the quality and safety of the drugs. End-to-end monitoring prevents the loss of thousands of dollars of perished products.




