Establishing a Drug Stability Budget During Storage and Transit
In previous blog posts, we have explored the fact that many drugs, vaccines and pharmaceutical products require refrigerated conditions in order to maintain efficacy. We also examined the importance of minimizing temperature excursions during transportation. Establishing a drug stability budget can minimize discards and ensure a quality product is delivered to the end-user.
We know that maintenance of correct storage temperature is important, but how do we go about quantifying and managing the stability of the drugs during transport. If we are able to do this, rather than arbitrarily disposing of drugs exposed to temperature excursions, you can minimize wastage through discarded drugs. So exactly how long can a pharmaceutical product be exposed to temperature excursion before its efficacy is compromised? The short answer is, it will depend on the pharmaceutical product and its chemical makeup. The amount of time the pharmaceutical product can spend outside of desired storage conditions is known as the Drug Stability Budget.
Determining a drugs shelf life

source: https://www.pharmoutsourcing.com/Featured-Articles/178206-Establishing-and-Managing-the-Drug-Product-Stability-Budget/
All drugs have a shelf life, its chemical composition determines how long it can be stored and maintain its stability. Over the course of the pharmaceutical product shelf life, the degradation can impact the efficacy, appearance, and even the safety of the product. Exposure to elevated temperatures through a temperature excursion can affect the rate of which the chemical composition is degraded. As with all chemical reactions, higher temperatures accelerate the rate of reaction. A rough guide is that the reaction rate doubles for every 10°C increase in temperature. When a substance is heated the particles are excited by the energy. This movement of the particles results in a higher rate of collisions. When particles collide the reaction can take place. Therefore, a higher temperature results in more excited particles, a higher rate of collisions and a faster reaction. However, it is not as straight forward as this. Simply increasing the rate of collisions is not enough. There is something known as the activation energy. This is the minimum amount of energy the particle must-have during a collision to get the reaction started. The diagram below shows this situation. The area under the curve is the number of particles that collide with sufficient energy to start the reaction. Those particles to the left of the activation energy threshold will not react, they simply bounce apart.
Understanding how temperature affects the rate of reaction is an important factor in understanding the shelf life of Pharmaceutical products and their tolerance to temperature excursions. There are many other factors to consider however, not only temperature. These include pH, light exposure, oxygen levels, ionic strength, molecular structure and moisture.
Drug Stability Tests
Stability tests should be performed on drugs, vaccines and other pharmaceutical products to establish the proper storage temperature, shelf life and the effects of elevated temperatures. The study should be run at the start of the products life-cycle. The tests should be performed with the product in its marketing packaging. It will study the effect of temperature, vibration, moisture and pressure on the product. The product should be stored in a strictly controlled environmental testing chamber and can go on for up to 36 months or more. Samples are extracted at regular intervals and tested. It is the results of these tests that are used to establish the manufacturers recommended guidelines on storage conditions and shelf life of the pharmaceutical product. They can also be used to determine the drug stability budget.
During manufacturing, pharmaceutical products are under strict controls. They are manufactured in compliance with regulatory guidelines and any temperature excursions are monitored and investigated. However, once the product leaves the manufacturing facilities the controlled environment is much less reliable. The vast majority of temperature excursions that result in discarded product occur during transport. If a strict 2-8°C temperature range was applied during transportation then it is highly probably no product would ever reach the end customer. Excursions during loading, unloading result in short-lived temperature excursions. This is why establishing a stability budget, the length of time the product can be exposed to a temperature excursion is important, known as the Time Out of Storage (TOS). The TOS will impact the product shelf life, and so it is a balance between what is a safe temperature excursion, the length of time the excursion takes place and its impact on the shelf life of the product. Establishing an acceptable TOS will decrease the product discard rate, and result in a more efficient supply chain, which can be extremely important in a pandemic where drug shortages can cost lives.
Before a pharmaceutical product reaches the patient, it has travelled through many layers of the pharma distribution channel. From the manufacturing facility to the distribution centers, to wholesalers and distributors and to the dispensing pharmacy. The travel between these destinations can be via road, air and sea. Pharmaceutical manufacturers realise the high probability of temperature excursions taking place at points throughout the cold chain distribution process. Therefore extended tests such as freeze-thaw cycles, temporary exposure to extreme temperatures between -20°C and +25°C for products normally stored between 2-8°C are conducted. The result of the tests gives a stability budget, the TOS that the pharmaceutical product can tolerate.
Drug Stability Budget
The drug stability budget refers to the products ability to withstand irreversible changes to its efficacy when exposed to temperatures above it’s normal, recommended, storage temperature. Through the extended testing performed by the pharmaceutical manufacturers, the rate of drug degradation is calculated. As mentioned above, for reactants to turn into a product, the activation energy must be exceeded. Arrhenius in his equation stated that :-
k = A e-Ea / R T
The equation gives the rate constant (k) of a chemical reaction at the absolute temperature (T) in Kelvin. Where A is the pre-exponential factor, Ea is the activation energy in J Mol-1 and R is the universal gas constant. The Arrhenius equation is used to establish an estimated shelf life at various temperatures for which extended shelf life studies have not been conducted.
Using mathematical simulations the impact of a temporary exposure of the product to higher or lower on the efficacy of the drug are calculated. These simulated results can also provide indicators as to the parameters that should be used for extended real-life testing.
Establishing the Drug Stability Budget through theoretical and practical experiments allow you to ensure product quality, safety and efficacy for the entire shelf life. It minimizes wastage by disposal of product. But it is only practical if the entire supply chain is monitored for temperature excursions. There must not only be a record of the temperature excursion but also the Time Out Of Storage (TOS) needs to be logged, and accumulated throughout the supply chain.
Every drug and vaccine may have a different TOS, which can result in a management nightmare for warehouses and logistics. Therefore, a simplified table of drug category and TOS can be used. This reduces the number of temperature sensors and data loggers needed. In a warehouse or cold chain, the product will typically be removed from this controlled storage conditions and be exposed in the loading bay to a temperature excursion. There may also be a period of time where the refrigerated truck needs to stabilize the temperature. The same will occur at the opposite end when being unloaded. Therefore, minimizing the number of transition points in the cold supply chain will be beneficial. The table below shows several categories of product and the typical TOS for each.
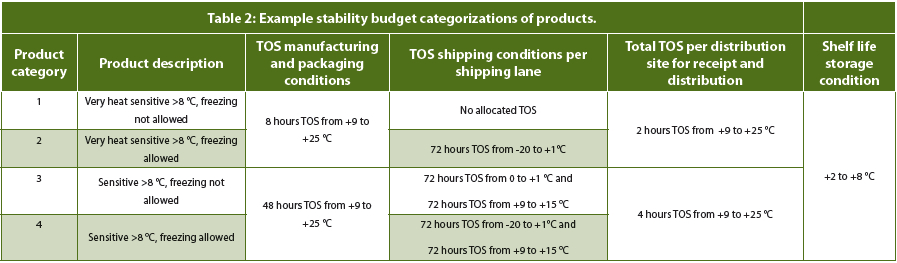
Source: https://www.pharmoutsourcing.com/Featured-Articles/178206-Establishing-and-Managing-the-Drug-Product-Stability-Budget/
Establishing the Drug Stability Budget, and assigning to TOS to each supply chain partner will allow you to maintain safety, quality and efficacy of the pharmaceutical product from manufacturing, through the supply chain to its shelf life. It also reduces the time spent investigating temperature excursions, accelerating the release and distribution of the product and minimizes discards.
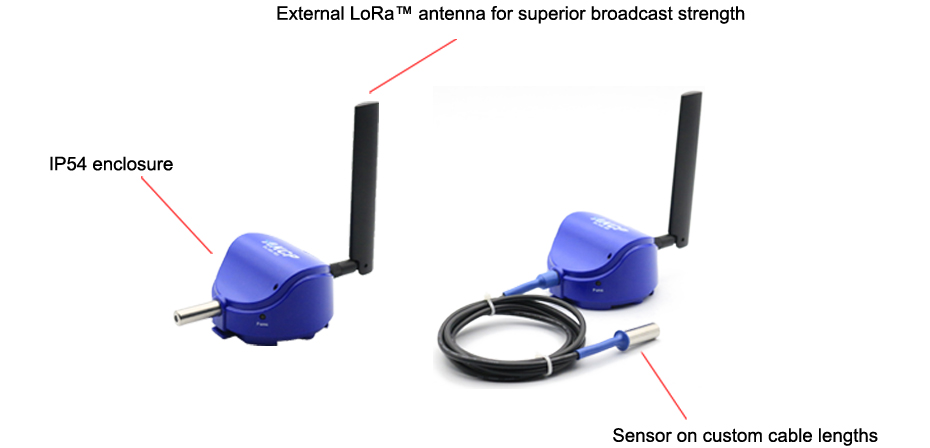
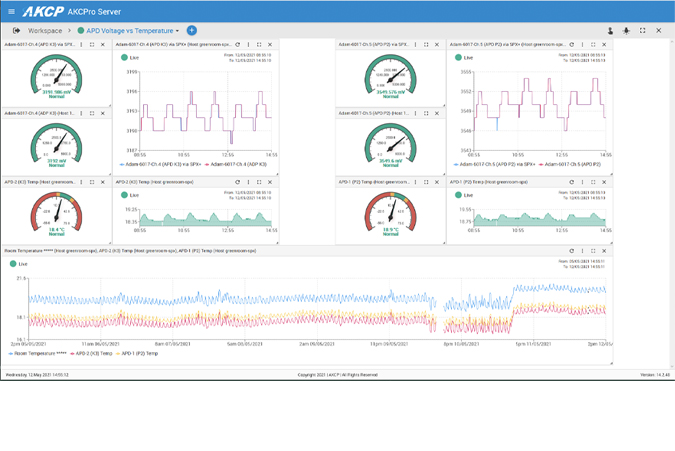
A suitable temperature monitoring system should be established throughout the supply chain to monitor and log temperature, humidity and provide an audited trail by way of a graph. Alerts during temperature excursions and accumulated TOS. AKCP provides Wireless temperature and humidity sensors, data loggers and complete software for reporting and graphing of the data.