Temperature Excursion Management in Pharmaceutical Storage
The quality of pharmaceuticals relies on environmental controls during their storage and handling. Every pharmaceutical item ought to be taken care of and stored under manufacturer-recommended storage conditions marked on the data information sheet or packaging.
Temperature Excursion Management in Pharmaceutical Storage is important during receipt of raw materials, manufacturing, and distribution of drugs.
System failures or human carelessness can cause circumstances leading to temperature excursions. The most significant environmental condition having the capacity to affect the nature of the pharmaceutical product is temperature. If the temperature excursions are not taken care of efficiently, there will be an adverse impact on product quality. There is a developing need to monitor for environmental excursions during pharmaceutical logistics to negate effects on the quality of the product. Quality Management System (QMS) should be implemented to avoid temperature deviations during the storage, transport, and distribution of pharmaceuticals.
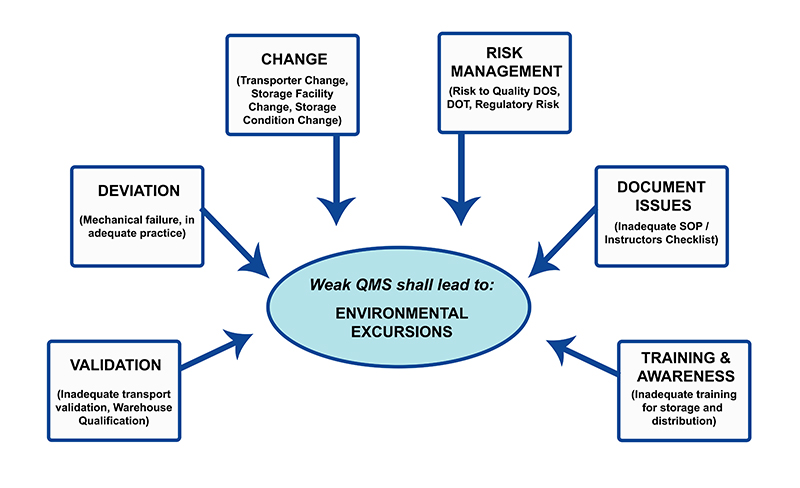
Pharmaceutical Temperature Excursion: An integrated approach for managing the quality system should include temperature excursion management. The overall temperature excursion management can be laid down in the following steps:
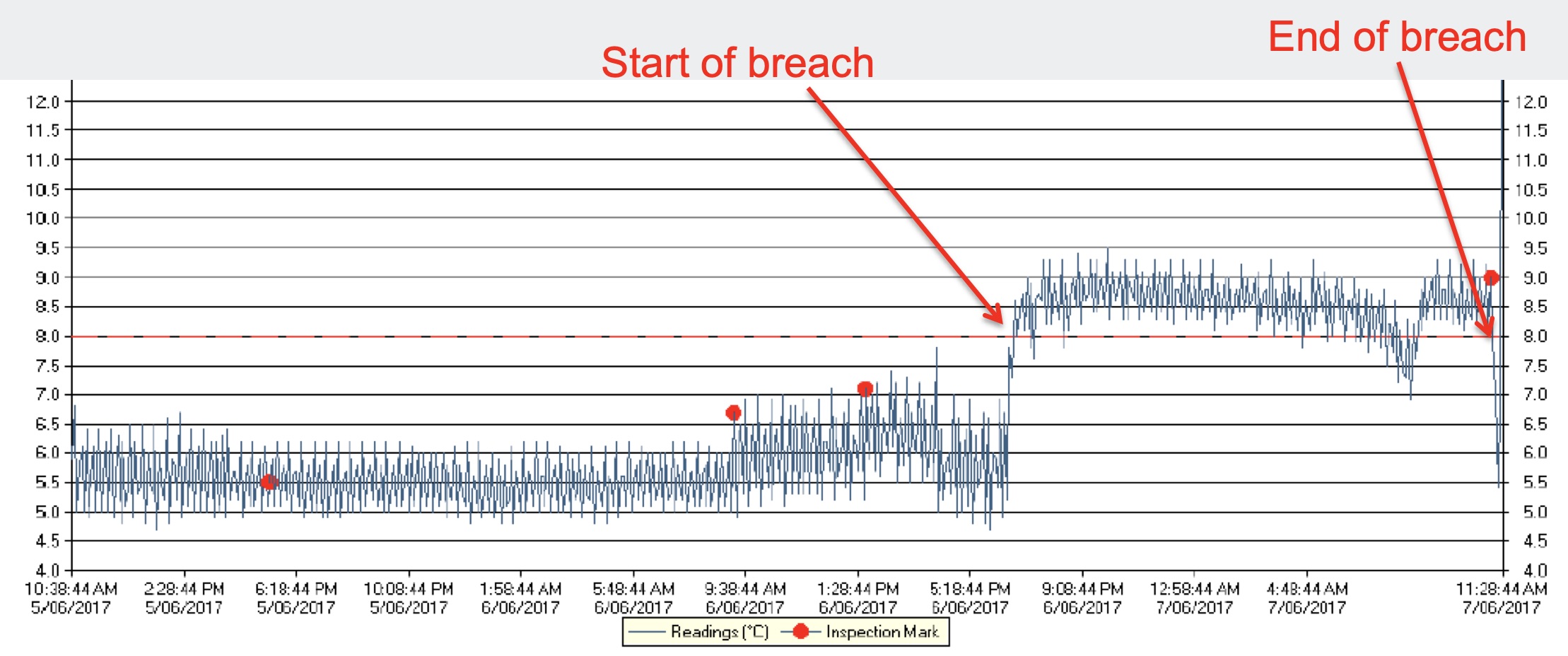
- Understanding temperature excursion: A temperature excursion as described by the World Health Organization (WHO) model guidelines is “an excursion event in which a Time Temperature-Sensitive Pharmaceutical Product (TTSPP) is exposed to temperatures outside the ranges prescribed for storage and/or transport. Temperature ranges for storage and transport may be the same or different; they are determined by the product manufacturer, based on stability data”. EU Good Distribution Practices (GDP) states under section 9.2, “The required storage conditions for medicinal products should be maintained during transportation within the defined limits as described by the manufacturers or on the outer packaging”. Studies indicate that exposure to products beyond specified environmental limits for a substantial time results in impurities and product quality degradation. Such compromises of products are not only undesired but also have can affect the efficacy of the pharmaceutical product and worse, result in an adverse reaction to the patient’s health.

- Storage temperature and humidity limits: Specified directions are stated for pharmaceutical products with reference to the temperature and humidity at which articles shall be stored, transported, and distributed. Pharmaceutical supply chain products require cold storage or climate-controlled storage to meet the manufacturer’s recommendations. For products from the class of cold chain, the storage condition is maintained at 2°C–8 °C. Similarly, the relative humidity shall be maintained below 60% and above 40% depending upon the hygroscopic nature of the product. When environmental stability data indicates there has been temperature deviation with storage and distribution at a lower or higher temperature and humidity there are protocols in place that may require the disposal of the product, or at the least taken out of circulation until tested and verified.
- Measurement devices: Temperature and Humidity measuring devices (popularly referred to as data loggers) are available. They log the temperature at a preset interval which will be downloaded to a computer system or QMS for review, evaluation, and recording. Periodic verification of the calibration status of temperature data loggers and upgrading of software is a prerequisite for uninterrupted and accurate information about product storage conditions. The temperature and relative humidity sensor should be placed on the hottest spot, concluded after temperature mapping of the area.
- Reason for Temperature excursion: In pharmaceutical factories and cargo areas, the required temperature is maintained with help of air handling units (AHU). The design and capacity of AHU are selected on the temperature required to be maintained.
Temperature excursions in a manufacturing area are caused due to the following reasons (not limited to):
- An inadequate number of air handling units (AHU) were installed to maintain the desired temperature conditions inside the manufacturing shop floor.
- Leakage or rupture from the air duct, resulting in an insufficient cooling effect.
- Mechanical failure in air handling unit (AHU). unprecedented temperature fluctuations.
- Power failure makes the AHU operation defunct.
- Lack of quality system and weak discipline of Good Manufacturing Practices (GMP) on the production shop floor.
- General awareness about the consequences of not maintaining the temperature within limits.
- Extreme weather changes and obsolete contingency plans to handle
Temperature excursions during transport are caused due to the following reasons:
- An unexpected delay in transportation due to which the temperature control cannot be maintained effectively
- Product pallets are kept in hot zones of airports or shipping yards.
- Reefer containers or refrigerated control vans are not deployed for transportation.
- The transport agency fails to maintain the planned transport condition.
- Higher cost to maintain the temperature within limits.
- Power failure due to short longevity of power bank during longer travel time.
- Good Distribution Practices (GDP) understanding amongst supply chain personnel about the adverse impact on product quality.
Consequences Of Temperature Excursion
The storage condition for the product is assigned based on scientific studies into the deterioration of the product during its life cycle. If the temperature excursion isn’t addressed immediately, the subsequent negative impacts are common:
- Loss of product.
- Decreased efficacy.
- Separation of layers in liquid products.
- Change in dissolution pattern of solid dosage.
- Discoloration of products.
Control of Temperature Excursions
To handle the temperature excursion strategic planning, effective packaging, and well-documented procedures are recommended. The development of a QMS database of pharmaceutical products is beneficial to assign quality storage conditions.
The storage conditions suitable for the product are assigned through the following tests:
At the merchandise development stage, the semi-finished product and final pharmaceutical product dosage are subjected to challenging conditions to monitor the potential impact on quality attributes.
- Hold time studies are administered to determine the allowable period of time at a specified storage condition without impacting quality. the standard attributes of product intermediates include chemical, microbiological and pharmacological determinants at various time points of stability.
- Accelerated condition stability study data of the product form an assurance for the storage condition that shall be suitable to product safety. The time stability data is generated in the laboratory to assess the product’s change in quality and attributes throughout the expiration date. The accelerated stability data is generated to gauge the impact on the quality of the product under a stressed condition.
- A freeze/thaw study for multiple cycles should be conducted to specify the effect of freezing, if any, and therefore the subsequent thawing. Samples from different layers (top, middle)
Thermal Packing During Transportations:
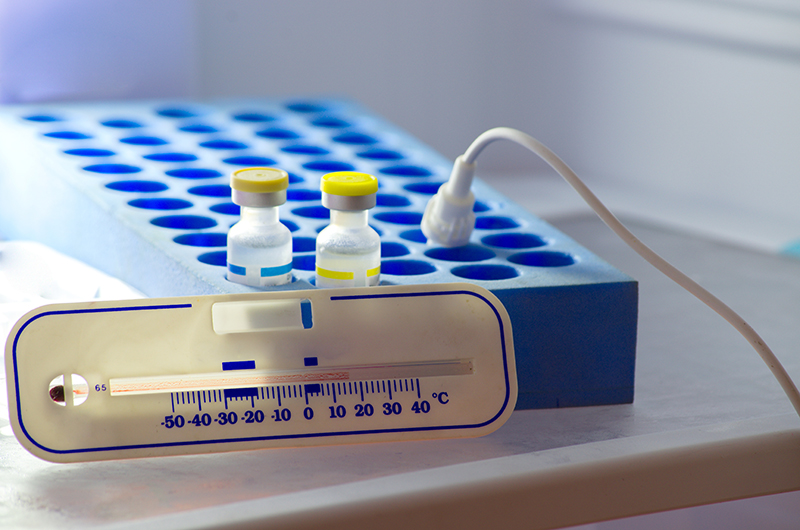
Packaging of pharmaceutical products for transport should include the supply of thermal packing and display of appropriate storage conditions. Caution notes to avoid storage outside the specified conditions shall help supply chain personnel protect the merchandise quality. The storage condition should be effectively displayed on the packaging of the pharmaceutical product. The packaging configuration card must contain the small print of knowledge loggers.
Procedure To Minimize Temperature Excursions: The temperature excursion has regulatory implications as well as an impact on business operations. A standard procedure (SOP) should be established and adherence ensured through adequate training to concerned personnel. An operational checklist of the Integrated Quality Management System (QMS) approach should include the qualification status of a manufacturing facility with special attention to environmental controls during storage and transportation.
Control of Temperature Excursion By Using AKCP Wireless Temperature and Humidity Sensor:
With AKCP wireless environmental monitoring solution, you can monitor temperature excursions with real-time alerts, data logging, and reporting.
A monitoring system gives an accurate picture of the temperature and humidity conditions of drug storage. Have confidence in the quality and safety of pharmaceutical products. End-to-end monitoring prevents the loss of thousands of dollars of perished products
Wireless Tunnel radio technology penetrates even thick secure storage and refrigerated cabinets. Battery-powered sensors with a 10-year battery life guarantee easy installation.