Vaccines play a vital role in society by preventing and eradicating various diseases. Newly developed vaccines such as pneumococcal conjugate vaccine (PCV) and rotavirus vaccines have the ability to save a million lives per year. The response to the 2019/2020 global COVID-19 pandemic resulted in mRNA ultra-low temperature vaccines being developed. It is essential for the efficacy of the vaccines that temperature monitoring devices are used to support the vaccine cold chain.
Vaccine research alone is not enough to improve immunization programs and overall public health. In order to safely administer the vaccine to a patient, proper handling throughout the cold chain should be employed to ensure a safe distribution. Many vaccines for different diseases have been rolled out, but cold chain systems are having difficulty in supporting immunization programs and ensuring the delivery of safe and potent vaccines. A vaccine undergoes a complicated journey throughout the supply chain. Since vaccines need to be kept at specific temperature ranges, the inability to properly regulate a vaccine’s temperature can cause a reduced potency, which results in delivering inadequate protection to patients against diseases. Improper cold chain logistics can also result in low availability of immunization supplies and inefficient use of resources.
Vaccine cold chain systems are vital in the successful administration of vaccines. Improving vaccine cold chain systems can further widen immunization coverage and reduce deaths caused by diseases that can be prevented through vaccines.
The staff needs to pay close attention to the cold chain to avoid the occurrence of temperature excursions. In order to support the staff in monitoring, Temperature Monitoring Devices (TMDS) can be used as a means of collecting more information regarding the temperature conditions inside refrigerator and freezer units.
Challenges of a Poor Vaccine Cold Chain
The cold chain is an uninterrupted temperature-controlled system that deals with the storage and distribution of temperature-sensitive items. It needs to keep vaccines at a temperature between 2°C and 8°C at all times (35°F and 46°F). It includes the cold storage unit at the manufacturing plant up to the transport and delivery of the vaccines to the respective healthcare facilities. It is vital that every stage in the cold chain vaccine is handled correctly to ensure that the vaccine does not lose its potency when its administered to the patient.
There are three main issues that hinder a cold chain’s optimum performance:
- Lack of cold chain storage capacity
- Need for the latest equipment
- Improper temperature monitoring and maintenance systems
Lack of Cold Chain Storage Capacity
Several factors lead to a lack of storage capacity in a cold chain, such as the need for routine immunization, campaigns, growth of population, and the introduction of new vaccines. There are also challenges that arise amid the delivery process. Space in the storage facility is created when there is an increased delivery frequency; however, this may incur an increase in delivery costs. It may also disrupt vaccine supply if vaccines are dispatched to sites based on storage capacity rather than need.
Insufficient capacity may be caused by poor visibility regarding the current status of cold chain equipment. A weak performance management system may also spell trouble for cold chain systems. Seldom routine checks and regular inventory updates might lead to a lack of consistent insight regarding cold chain performance, which is a crucial step in ensuring that the potency of the vaccines is maintained throughout the system.
The ability to anticipate future needs is also vital, considering the lead times needed for the procurement and installation of cold chain equipment, which can take up to 1.5 to 2 years. Capacity needs to expand in proportion to the development of new vaccines.
Improper Monitoring and Maintenance Systems
Overexposure to extreme temperature changes at any stage in the cold chain can potentially harm vaccines. Unstable conditions can render a whole batch of vaccines ineffective, causing financial loss and product wastage. Cold chain errors may also require a patient to get revaccinated, which has societal repercussions if it led to a massive loss of confidence in immunization programs among the general public brought by ineffective vaccines.
Using certain types of TMDs, like bi-metal stem TMDs, food TMDs, infrared TMDs, alcohol or mercury thermometers, and other TMDs that do not have a valid Certificate of Calibration Testing can be difficult to read since they are only able to check the temperature at the exact time they are used. TMDs not explicitly made for cold chain monitoring may not be able to detect when temperatures fall outside the required range.
The below graph illustrates the different temperatures monitored at various positions in and around a medical refrigerator. The Glycol Mid sensor captured the most stable curve. Use of glycol as a buffer against rapid fluctuations of air temperature helps to eliminate false positives.
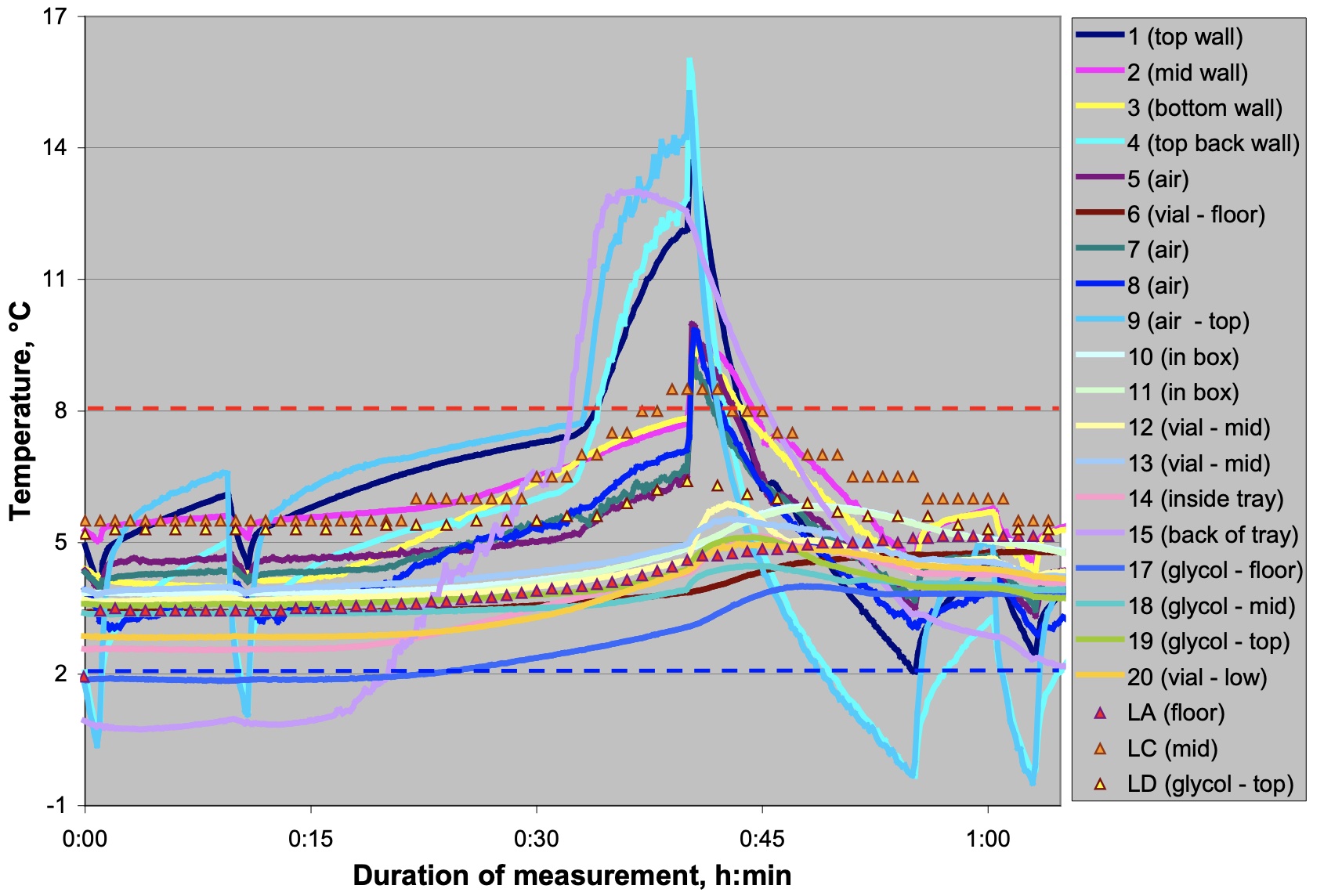
Chojnacky, M (2017) Cold Chain Management: Temperature Monitoring Solutions National Institute of Standards and Technology. Temperature, Pressure, and Flow Metrology Division Gaithersburg, MD
The World Health Organization states that all vaccine cold chain equipment should have Continuous Temperature Monitoring Devices (CTMDs) to monitor properly any excursions that might occur. Unfortunately, some systems use stem thermometers, which are unable to detect such temperature changes. CTMDs enable increased visibility into fridge performance, which allows staff to take the necessary action if such a need arises. Meanwhile, higher-level sites may need remote TMDs, which gives additional security by means of email alarms and SMS. In order to fully utilize CTMDs, the assigned staff should know the necessary procedures to resolve warnings. Competent and adequately trained staff should also be dispatched and available to troubleshoot or repair TMDs in the event of severe freeze and heat events.
Need for the Latest Equipment
Outdated units usually have poor temperature control capabilities and lack freeze protection. These old units may endanger vaccines and hinder the cold chain from performing efficiently. Temperature damage is often caused by equipment failure, which can be prevented with the proper alarm and monitoring system.
Domestic fridges are relatively cheaper and widely available; however, they are not conducive for vaccine storage and are unable to properly maintain the required temperature ranges. Absorption-type fridges are fueled by bottled liquid petroleum or kerosene; however, they are now prohibited to use by the WHO since they pose a risk to proper delivery and storage.
Geographic Challenges
Many low-income countries are having difficulties in facilitating a smooth vaccine delivery system due to limitations in their capability to meet the immunization program requirements. Many rural areas in the globe do not possess the necessary power supplies to ensure the proper maintenance of vaccines. A power outage can disrupt the cold chain, which can result in a massive batch of ineffective vaccines that need to be discarded. Geography and the wide-scale demand for vaccines is one of the biggest challenges faced by the cold chain since it needs to sutain the entire population. Service delivery types are also challenging for these countries since they provide more than 50% of immunization programs via outreach. Such a delivery method takes a toll on health care workers who might need to walk long distances in order to dispatch the vaccines. Additionally, it may also have an effect on the frequency of delivering immunization sessions.
The diverse population settlements lead to a proliferation of specialized contextual challenges to cold chain capabilities. In Uganda, 70% of healthcare facilities have limited road networks, which makes it hard to access off-grid solutions like gas.
How to Fortify Vaccine Cold Chain
In order to strengthen the cold chain system, the following recommendations can be considered.
For limited cold chain storage capacity
- Gaps in capacity based on current and future needs should be determined
- Proper resource mobilization
- Accurate monitoring plan during implementation
For temperature changes and equipment malfunction
- Establishment of temperature monitoring and control devices usage and practices
- Increased competence and availability of assigned equipment technicians
- Guaranteed availability of spare parts
For cold chain equipment
- Ensure the cold chain system is equipped with upgraded and relevant equipment
- Full utilization of tools to properly understand the need for equipment trad-offs
- Regular conversation with suppliers to ensure proper equipment selection
It is crucial to use purpose-built vaccine refrigerators and the necessary temperature monitoring devices to achieve reliable standards of cold chain maintenance. Investing in new cold chain equipment can save more money in the long run, considering the average 10-year life span of the equipment.
The Role of a Digital Data Logger
In the Vaccine Storage and Handling Toolkit published by the Centers for Disease Control and Prevention intended to aid providers in the proper storage and handling of vaccine supply, a specific type of TMD was recommended for continuous temperature monitoring — the Digital Data Logger (DDL).
Using DDL devices for temperature monitoring allows vaccines to be monitored more closely than standard thermometers. It allows for the reading rate or logging interval to be programmed within the system, which leads to a better temperature recording and measurement. Results can also be immediately analyzed through the use of DDL, which eliminates time-consuming paperwork. All thermal excursions are recorded, which strengthens confidence in vaccine supply efficacy. It also shows a simplified reading through the removal of false alarms, and it eliminates the need to frequently download the recorded readings through archival data. When the temperature range falls out of range, it sends a notification to staff members, which allows them to immediately respond to the situation. Real-time monitoring is also possible through wireless DDL models. Lastly, the DDL enhances the ability of the staff to know when the device needs to have a battery change.
AKCP provides NIST calibrated sensors for the pharmaceutical industry. Optional glycol jar attachment can be supplied. The sensor will act as a data logger when not in the range of a gateway. When a gateway is within range it will connect, upload stored data and provide real-time monitoring and alerts through the Wireless Tunnel™ Cloud platform.
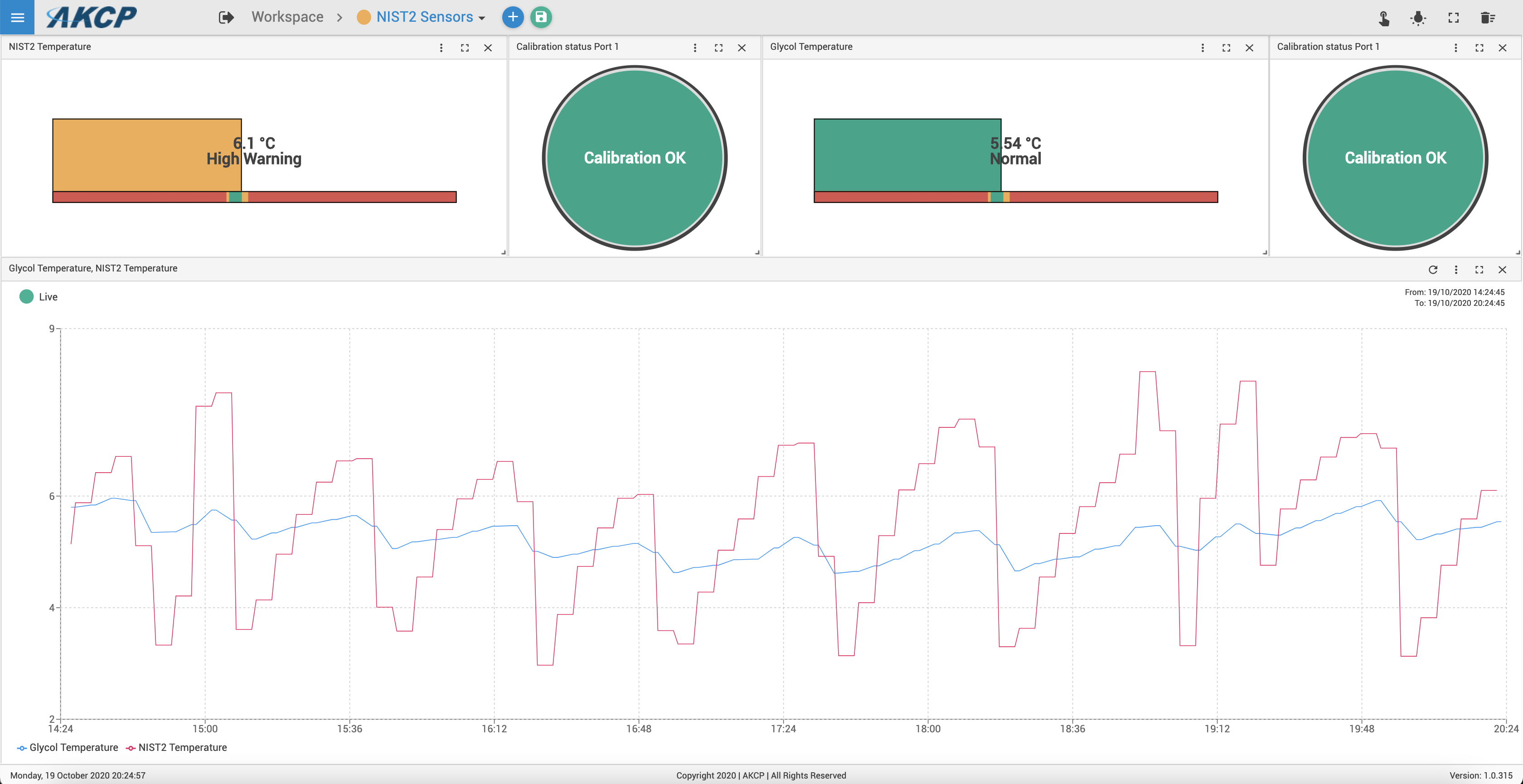
NIST2 sensors in a refrigerator. Redline denotes sensor reading air temperature, the blue line is a sensor in glycol.
Reference Links:
https://jl-alonso.medium.com/using-temperature-monitoring-devices-to-improve-the-cold-chain-for-vaccines-45cb63032ea2
https://www.researchgate.net/publication/308535283_Improving_cold_chain_systems_Challenges_and_solutions
https://theconversation.com/cracking-the-cold-chain-challenge-is-key-to-making-vaccines-ubiquitous-99329




